One of the most critical questions the world is waiting for an answer is if the human body can mount a protective response that provides a lasting immunity against SARS-CoV-2. This understanding is crucial to the success of vaccines, the successful return of recovered patients to their pre-pandemic lifestyle, and also, how readily the world can overcome the fear of pandemic spread.
Sporadic reports of Covid-19 recovered people falling ill and testing positive for the disease again — have stoked fears of re-infection due to a short immune memory. Matters have only got worse because of the discouraging predictions made by the mainstream media.
However, the latest communication from the Indian Council of Medical Research (ICMR) says
- There has been no evidence so far of repeat COVID-19 infections or relapses in recovered patients.
- All the supposed cases of repeat infection from some states may be post-viral symptoms of Covid-19 that still need to be studied thoroughly.
- The SARS-CoV-2 virus seems to be persisting in some individuals, but after ten days, it's unlikely to spread & infect others.
- The commonly performed tests to diagnose Covid-19 can identify persistent viral particles, but can't say if they are live or inactivated.
- To establish SARS-CoV-2 re-infection, one needs to show a positive live virus in a BSL-3 level lab. Epidemiologists world over share a similar view.
Let's analyse the scientific evidence that has emerged recently in this regard.
First question: Is there any evidence that shows the anti-SARS-CoV-2 antibodies are neutralising enough to be called protective?
A recent study (MedRxiv, 20 July 2020) showed that the presence of antibodies against the Receptor Binding Domain (RBD) region of the spike (S) protein was highly predictive of SARS-CoV-2 infection. The IgG antibodies targeting SARS-CoV-2 RBD showed high specificity (100%) and sensitivity (97%) against the infection after 14 days from onset of illness. However, in comparison, different antibodies showed varying durations of persistence. The IgG seropositivity was sustained in patients up to 75 days (last time point measured). Still, the IgM and IgA responses were short-lived (many individuals seroreverted within two months of the onset of illness).
The authors identified neutralising IgG antibodies as a possible correlate of protective immunity. While the association between RBD-IgG with neutralising titers and the persistence of these antibodies at late time points is encouraging, further work is needed to define the optimal antibody-mediated correlates of protective immunity. The authors, mentioning the limitations of the study, stated that the study does not provide information about whether the immune response protected the individuals from subsequent infection.
Is there any direct human evidence that the anti-SARS-CoV-2 neutralising antibodies are protective against SARS-CoV-2 infection "in humans"?
Our understanding of lasting immunity and successful vaccine development against SARS-CoV-2 would be significantly facilitated by the identification of immunological parameters that correlate with protection in humans. However, to date, most studies available on protective immunity had been performed in animal models, and correlates of protection had not been established in humans. A recent study provided the first direct evidence that anti-SARS-CoV-2 neutralising antibodies protect against SARS-CoV-2 re-infection in humans.
This new study (MedRxiv, 13 Aug 2020) done by Greninger lab at the University of Washington School of Medicine in Seattle describes a SARS-CoV-2 outbreak reported on a fishing vessel that was associated with a high attack rate (>85%).
One hundred twenty-two sailors went on a ship, out of which 6 had anti-SARS-CoV-2 antibodies before starting the trip. Only three crew-members tested seropositive before the boat's departure in initial serological screening and also had neutralising and spike-reactive antibodies in follow-up assays. Then 104 people out of 122 got infected on the ship. Metagenomic sequencing of 39 viral genomes suggested the outbreak originated mostly from a single viral clade.
However, the three crew members with prior neutralising antibodies did not become PCR positive. None of these three crew-members with neutralising antibody titers showed evidence of any bona fide viral infection or experienced any symptoms during the viral outbreak. Therefore, the authors conclude that the presence of neutralising antibodies from a prior infection was significantly associated with protection against re-infection.
The study- a non-peer-reviewed preprint has several limitations (can be read in full article), but even with such a high attack rate (>85%), the lack of infection in those three crew- members with neutralising antibodies was statistically significant in comparison the rest of the boat's crew.
Overall, the study (famously known as the Seattle Boat Study) provides the first direct evidence that anti-SARS-CoV-2 neutralising antibodies are protective against SARS-CoV-2 infection in humans. This evidence is encouraging as we wait for more studies.
Introduction to immunological mechanisms before we go further:
Before we go to the next study, let's summarise what we know about the protective immune responses that the body mounts to fend off a re-infection.
The human body makes use of two arms of the immune system to fend off viral invaders that keep returning.
- B cells that produce antibodies that bind to the virus. It also saves some memory in the form of long-lived memory B cells that are ready to act if the virus comes back.
- T cells, which guard the body checking and destroying the infected cells, disrupt the virus's ability to replicate. Even T cells can also endure for years.
Immunological memory:
In some studies, the antibody levels reduced to practically undetectable levels within about three months and many as misinterpreted it as loss of immunity. We should know that it's very natural for the antibodies to wane once the virus disappears. Also that even those very low levels of antibodies are enough to provide protective immunity - this means that even if antibody levels drastically reduce and reach low levels, the immune system often has a backup plan. The memory B cells, which linger in the bone marrow until a virus returns, attain a new identity and become plasma cells to produce specific antibodies.
Another pertinent concept is long-lasting T cell immunity: An article published in Nature, 2002 showed how memory T cells induced by previous pathogens could shape susceptibility to, and the clinical severity of, subsequent infections.
SARS-CoV of 2003 induced T cells against the virus, so long-lasting that they have been found 17 years after infection.
A recent study published in Nature, 15 July 2020 showed that infections with beta coronaviruses induce multi-specific and long-lasting T cell immunity against the structural N protein. In this study, the researchers evaluated T cell responses against the structural (nucleocapsid (N) protein) and non-structural (NSP7 and NSP13 of ORF1) regions of SARS-CoV-2 in 36 individuals convalescing from COVID-19. All these individuals had developed CD4 and CD8 T cells that recognised multiple regions of the N protein.
Next, the authors showed memory T cells that are reactive to the N protein of SARS-CoV in 23 patients who had recovered from SARS-CoV infection of 2003. These cells found 17 years after the outbreak of SARS in 2003) were not only long-lasting but also displayed robust cross-reactivity to the N protein of SARS-CoV-2.
Understanding the distribution, frequency and protective capacity of pre-existing structural or non-structural protein-associated SARS-CoV-2 cross-reactive T cells could be necessary for the explanation of some of the differences in infection rates or pathology observed during this pandemic.
Common Cold coronavirus and SARS-CoV-2 fragments that are genetically similar show cross-reactivity via memory T cells. In some individuals, pre-existing T cell memory against common cold coronaviruses can cross-recognise SARS-CoV-2, down to the exact molecular structures, which could help us understand why some people show milder symptoms of Covid-19, while others get severely sick. Read more here.
A study published in Cell, May 2020 evaluated T -cell responses to SARS-CoV-2 coronavirus in humans infected with COVID-19 and the unexposed individuals. The authors demonstrated memory CD4 T cells and CD8 T cells detected in 100% and 70% of patients who recovered, respectively. Also, they detected SARS-CoV-2-reactive CD4 T cells in ∼40%-60% of unexposed individuals, which suggested cross-reactive T cell recognition between circulating "common cold" coronaviruses and SARS-CoV-2.
For more details, one can refer to this article on Researchgate that explains how there has been a paradigm shift from B- cell responses to T- cell responses in our approach to SARS-CoV-2.
Is there any evidence that suggests the human body produces robust memory to counter re-infection?
A recent study published by Karolinska University in Cell, 11 Aug 2020 showed that SARS-CoV-2-specific memory T cells would be critical for long-term immune protection against COVID-19. Researchers mapped the functional and phenotypic landscape of SARS-CoV-2-specific T cell responses in different groups of people - unexposed individuals, exposed family members, and individuals with acute or convalescent COVID-19.
- Acute phase SARS-CoV-2-specific T cells displayed a highly activated cytotoxic phenotype that correlated with various clinical markers of disease severity.
- Convalescent-phase SARS-CoV-2-specific T cells were polyfunctional and displayed a stem-like memory phenotype.
- Importantly, SARS-CoV-2-specific T cells were detectable in antibody-seronegative exposed family members and convalescent individuals with a history of asymptomatic and mild COVID-19.
Collectively, the data showed that SARS-CoV-2 elicits robust, broad and highly functional memory T cell responses, suggesting that natural exposure or infection may prevent recurrent episodes of severe COVID-19.
Does this memory get better with time?
A study published in MedRxiv, 15 Aug 2020 longitudinally assessed a group of individuals, who had recovered from mildly symptomatic COVID-19, to determine if they develop and sustain immunological memory against SARS-CoV-2. The study found that recovered individuals developed SARS-CoV-2-specific IgG antibody and neutralising plasma, as well as virus-specific memory B and T cells that not only persisted but in some cases increased numerically over three months following symptom onset.
The next level of the study was to check how these mildly symptomatic but recovered individuals would respond when they re-encounter the virus - whether they will display antiviral protective immunity or not. The study showed that the SARS-CoV-2-specific memory lymphocytes exhibited characteristics associated with potent antiviral immunity:
- memory T cells secreted IFN-γ and expanded upon antigen re-encounter,
- memory B cells expressed receptors capable of neutralising virus when expressed as antibodies.
These findings demonstrate that even mild COVID-19 elicits memory lymphocytes that persist and display functional hallmarks associated with antiviral protective immunity.
Although the study found memory B cells capable of producing neutralising antibodies that recognise SARS-CoV-2, we need more studies because these cells are more challenging to locate and count than antibodies — but thus far, the evidence suggests that they proliferate.
Is the immunity durable?
An extensive serological study published in MedRxiv, 16 Aug 2020 quantified population-level exposure and defined the correlates of immunity against SARS-CoV-2. The researchers found that the severe Covid-19 cases (compared to mild COVID-19 ones) exhibited higher virus-neutralising titers and antibody levels against three regions - nucleocapsid (N), the receptor-binding domain (RBD) and the S2 region of the spike protein. All cases, including the asymptomatic individuals, seroconverted by two weeks post-PCR confirmation. Two neutralising antibody titers - those against RBD- and S2-specific remained elevated and stable for at least 2-3 months post-onset. In contrast, those against N were more variable with rapid declines in many samples. In contrast to other reports, this report concluded that immunity is durable for at least several months after SARS-CoV-2 infection.
The CDC controversy:
Quoting the evidence, The Centres of Disease Control and Prevention (CDC), stated in their recent (16 Aug 2020) guideline that very low levels of SARS-CoV-2 virus could stick around in people's systems for up to three months, so there is no need to get tested again in that timeframe unless new COVID-19 symptoms develop. However, if a person who has recovered from COVID-19 develops symptoms again within three months, he should get evaluated for re-infection and isolate himself.
But mainstream media misinterpreted this statement as "People who have been infected with COVID-19 and recovered are protected for up to three months and can safely interact with others, according to new CDC guidance."
A backlash came from Carl T. Bergstrom (@CT_Bergstrom) Professor in Biology, the University of Washington, "CDC guidelines say that people are protected for *at least* three months, not *up to* three months." in his twitter thread that offers a good explanation on the CDC guidelines.
His thread says "There is nothing at all in the CDC statement about evidence that immunity wanes after three months. Rather, they are making a recommendation based on positive evidence that immunity doesn't wane before three months. The 3- month window is driven by the availability of evidence. This is a new virus. We haven't had enough cases here in the US that are four or five months old to have had time to determine whether you get re-infection after that duration."
Comparison of SARS-CoV-2 with other viruses and relevance to vaccine development:
HIV: We all, including the vaccine developers, want a long-term immunity & that too, a sterilising immunity (antibody-mediated) that can rapidly prevent a returning virus from infecting the body. However, not all vaccines or infections elicit the neutralising antibodies needed to induce a sterilising immunity. HIV infection rarely produces neutralising antibodies; that's why we don't have vaccines against HIV. Read more here
Influenza Virus: SARS-CoV-2 does not seem to mutate as rapidly as the influenza viruses, which is an encouraging finding for the success of vaccines.
Conclusion:
The evidence is very clear that people are not getting reinfected at least in the short term. CDC says there are no confirmed reports to date of a person being reinfected with COVID-19 within three months of initial infection. Since it is something that we need to follow long term, it is too early to give any definitive statement. Although we cannot predict how long this protective immunity will last, researchers are optimistic about the emerging data that the human immune system will most likely fend off SARS-CoV-2 if exposed to the virus again.
Recommended read: What the immune response to the coronavirus says about the prospects for a vaccine. Click here to read.
Tags:
We present five studies published till now, which show that a single dose of vaccine may be enough to elicit a robust immune response in people previously ...
The possible benefits by which one can use mobile applications (apps) for contact tracking in managing the COVID-19 pandemic have been discovered. Various ...
LEIPZIG, Germany: Given that little time has passed since the outbreak of SARS-CoV-2, it comes as no surprise that only sparse data on COVID-19 is available...
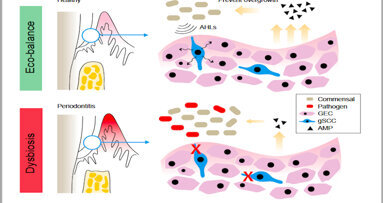
PHILADELPHIA, U.S./CHENGDU, China: Periodontitis remains the sixth most prevalent infectious disease worldwide, and the most common cause of tooth loss ...
SASKATOON, Saskatchewan, Canada: Researchers from the University of Saskatchewan (USask) in Saskatoon are currently working on two large projects that have ...
As the COVID-19 vaccination drive continues to expand throughout the world, SARS-CoV-2 variant mutations and breakthrough infections have posed a new public...
This guest editorial by Dr Sanjiv Hyoju talks about a possible connection between a diet-induced disturbance in the gut microbiome equilibrium and its ...
Mind your mind because a dilapidated one is worse than having none. Dr. Bhavdeep Singh Ahuja emphasizes the importance of mental health in these challenging...
NEW YORK, US: Seeking to better characterise the inflammatory consequences of periodontal inflammation, researchers have recently developed two scores to ...
Do you know that there are 14 already established pieces of scientific evidence to show a direct association between VitaminD deficiency & COVID19 ...
Live webinar
Tue. 24 February 2026
11:30 pm IST (New Delhi)
Prof. Dr. Markus B. Hürzeler
Live webinar
Wed. 25 February 2026
1:30 am IST (New Delhi)
Prof. Dr. Marcel A. Wainwright DDS, PhD
Live webinar
Wed. 25 February 2026
9:30 pm IST (New Delhi)
Prof. Dr. Daniel Edelhoff
Live webinar
Wed. 25 February 2026
11:30 pm IST (New Delhi)
Live webinar
Thu. 26 February 2026
6:30 am IST (New Delhi)
Live webinar
Tue. 3 March 2026
9:30 pm IST (New Delhi)
Dr. Omar Lugo Cirujano Maxilofacial
Live webinar
Wed. 4 March 2026
6:30 am IST (New Delhi)
Dr. Vasiliki Maseli DDS, MS, EdM



 Austria / Österreich
Austria / Österreich
 Bosnia and Herzegovina / Босна и Херцеговина
Bosnia and Herzegovina / Босна и Херцеговина
 Bulgaria / България
Bulgaria / България
 Croatia / Hrvatska
Croatia / Hrvatska
 Czech Republic & Slovakia / Česká republika & Slovensko
Czech Republic & Slovakia / Česká republika & Slovensko
 France / France
France / France
 Germany / Deutschland
Germany / Deutschland
 Greece / ΕΛΛΑΔΑ
Greece / ΕΛΛΑΔΑ
 Hungary / Hungary
Hungary / Hungary
 Italy / Italia
Italy / Italia
 Netherlands / Nederland
Netherlands / Nederland
 Nordic / Nordic
Nordic / Nordic
 Poland / Polska
Poland / Polska
 Portugal / Portugal
Portugal / Portugal
 Romania & Moldova / România & Moldova
Romania & Moldova / România & Moldova
 Slovenia / Slovenija
Slovenia / Slovenija
 Serbia & Montenegro / Србија и Црна Гора
Serbia & Montenegro / Србија и Црна Гора
 Spain / España
Spain / España
 Switzerland / Schweiz
Switzerland / Schweiz
 Turkey / Türkiye
Turkey / Türkiye
 UK & Ireland / UK & Ireland
UK & Ireland / UK & Ireland
 International / International
International / International
 Brazil / Brasil
Brazil / Brasil
 Canada / Canada
Canada / Canada
 Latin America / Latinoamérica
Latin America / Latinoamérica
 USA / USA
USA / USA
 China / 中国
China / 中国
 Pakistan / Pākistān
Pakistan / Pākistān
 Vietnam / Việt Nam
Vietnam / Việt Nam
 ASEAN / ASEAN
ASEAN / ASEAN
 Israel / מְדִינַת יִשְׂרָאֵל
Israel / מְדִינַת יִשְׂרָאֵל
 Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
 Middle East / Middle East
Middle East / Middle East















































To post a reply please login or register